The clinical trials industry in India is growing rapidly, and competition for Clinical Research Coordinator (CRC) roles is increasing every year. If you are planning to apply for this role, your clinical research coordinator resume must clearly show that you are ready to manage real-world clinical trials.
Recruiters spend only 6–8 seconds scanning a resume. In that short time, your resume should highlight your training, technical skills, certifications, and understanding of compliance. In this guide, we will walk you through resume structure, keyword strategy, tips for freshers, and how choosing the right clinical research course can strengthen your profile.
Why Your Clinical Research Coordinator Resume Is Important
Your resume is your first professional introduction. Before you step into the interview room, your clinical research coordinator resume speaks for you.
A strong resume shows:
- Knowledge of clinical trial processes
- Understanding of GCP and regulatory compliance
- Experience in documentation and patient coordination
- Technical and communication skills
Companies in clinical research value precision and accuracy. If your resume is well-structured and detailed, it reflects the same qualities recruiters expect from a CRC.
At LLRI, our skilled experts help you throughout the way till you get your first job in clinical research, from resume building to in-depth theoretical knowledge. Come and join one of the best clinical research courses to take a step towards a bright future.
What Does a Clinical Research Coordinator Do?
Before writing your resume, you must understand the role clearly. It is important to know the ins and outs of this role so that you can thoroughly optimize your resume to best demonstrate your skill set.
A Clinical Research Coordinator typically:
- Manages day-to-day clinical trial activities
- Coordinates with investigators, sponsors, and CROs
- Handles patient recruitment and informed consent
- Maintains documentation and regulatory records
- Ensures compliance with trial protocols
Since CRCs play a key role in maintaining trial quality, companies carefully review every clinical research coordinator resume before shortlisting candidates.
Best Resume Format for Clinical Research Coordinator
Choosing the right resume format for clinical research coordinator roles improves readability and keyword visibility.
Follow this structure given below for the best outcomes:
- Header and Contact Details
Include:
- Full Name
- Mobile Number
- Professional Email ID
- LinkedIn Profile
Keep it clean and professional.
- Clinical Research Coordinator Resume Objective
Your clinical research coordinator resume objective should clearly mention your career goal and relevant skills.
Example:
“To secure a position as a Clinical Research Coordinator where I can apply my knowledge of GCP guidelines, patient coordination, and clinical documentation to support ethical and high-quality clinical trials.”
For freshers, this section is very important because it highlights your intent and readiness.
- Professional Summary (For Experienced Candidates)
If you have experience, include:
- Total years of experience
- Therapeutic areas handled (Oncology, Cardiology, etc.)
- Major responsibilities
- Key achievements
This section strengthens your clinical research coordinator resume by showing practical exposure.
- Education & Clinical Research Course Details
List your qualifications in reverse chronological order.
Example:
Post Graduate Diploma in Clinical Research – Learning Labb Research Institute (LLRI), Pune (2023)
Bachelor of Pharmacy – Mumbai University (2021)
If you completed training from a reputed clinical research training center like LLRI, mention course highlights. Many recruiters prefer candidates from structured programs with practical exposure.
- Certifications & Training
Adding certifications improves keyword optimization and credibility.
Include:
- GCP Certification
- ICH Guidelines Training
- Clinical Data Management
- Clinical Trial Monitoring Basics
If relevant, you may mention that the clinical research course fees were affordable and industry-oriented, especially if the institute has strong placement support.
- Technical & Soft Skills
Recruiters search resumes using keywords. Your clinical research coordinator resume must include both technical and soft skills.
Technical Skills:
- CTMS & EDC Software
- Case Report Form (CRF) Handling
- Regulatory Documentation
- Patient Recruitment & Consent
- Microsoft Excel & Documentation Tools
Soft Skills:
- Attention to detail
- Communication skills
- Team coordination
- Time management
- Problem-solving
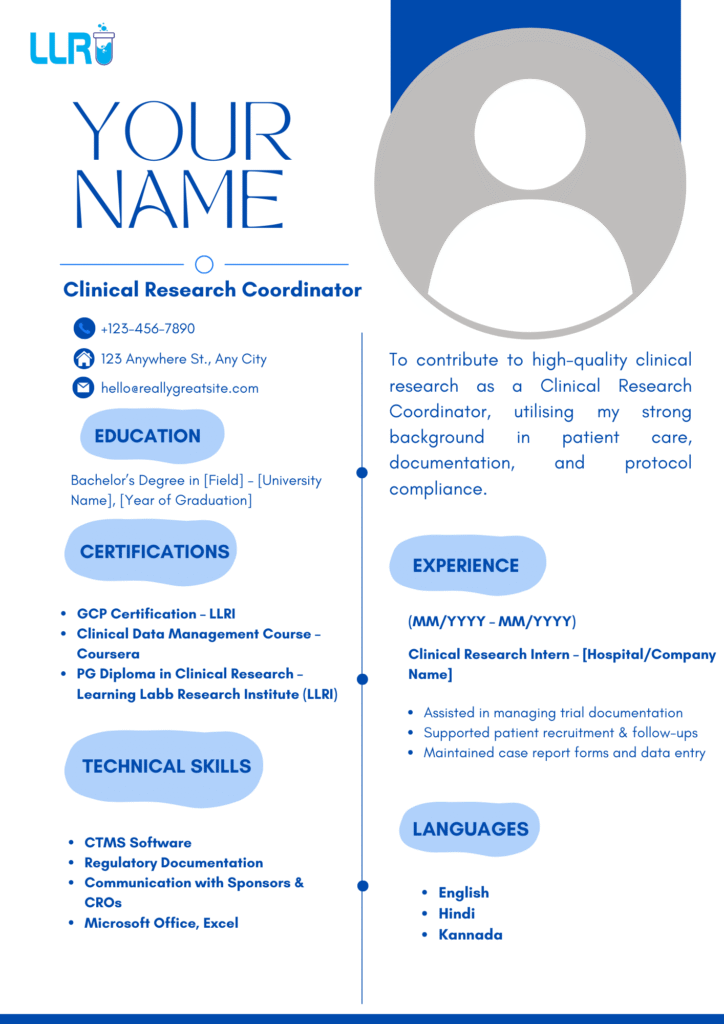
- Sample Resume for Coordinator Position
Here is a short sample resume for coordinator position:
Name
Email | Phone | Location
Objective:
To contribute to clinical research projects as a Clinical Research Coordinator by applying my knowledge in GCP compliance, documentation, and patient management.
Education:
PG Diploma in Clinical Research – LLRI, 2023
Bachelor of Pharmacy – 2021
Experience:
Clinical Research Intern – Apollo Hospitals (2023)
Assisted in documentation management
Coordinated patient follow-ups
Maintained CRFs and trial records
Skills:
CTMS | Regulatory Documentation | GCP | MS Excel
This simple and structured format improves readability and searchability.
Entry Level Clinical Research Coordinator Resume Tips
If you are creating an entry level clinical research coordinator resume, focus on:
Internships
- Academic projects
- Certifications
- Practical training exposure
- Strong resume objective
Freshers should use a clean and clear resume format for clinical research coordinator roles. Avoid unnecessary information and focus on relevant training.
Why Training Matters: Choosing the Right Institute
A professional certification can significantly strengthen your clinical research coordinator resume. Institutes like Learning Labb Research Institute (LLRI) are known for:
- Industry-focused curriculum
- Practical training
- Placement assistance
- Affordable clinical research course fees
Choosing a reputed clinical research training center improves both knowledge and credibility during interviews.
How to Optimise Your Clinical Research Coordinator Resume for Job Portals
If you are applying through Naukri, LinkedIn, or Indeed:
- Use the keyword clinical research coordinator resume naturally throughout your profile
- Include related terms like entry level clinical research coordinator resume and resume format for clinical research coordinator
- Mention clinical research course certifications clearly
- Keep formatting ATS-friendly (simple fonts, no complex graphics)
- Recruiters often filter resumes using keywords. If your resume is not optimized, it may not appear in searches.
Final Checklist Before Submitting Your Resume
Before sending your clinical research coordinator resume, check:
- Clear objective
- Proper structure
- Relevant keywords included
- Certifications mentioned
- No spelling or grammar errors
- Professional formatting
- Small mistakes can reduce your chances of shortlisting.
The Bottom Line
A powerful clinical research coordinator resume is not just a document — it is your entry pass into the clinical trials industry. Whether you are preparing an entry level clinical research coordinator resume or updating your profile with experience, the goal is the same: show that you are trained, compliant, and ready for responsibility.
Invest in the right clinical research course, build your technical skills, and present them clearly on paper. A well-crafted resume backed by proper training can open doors to hospitals, CROs, and pharmaceutical companies across India.
Your career in clinical research begins with one strong step — a resume that speaks confidence, compliance, and competence. Take the time to build it right, optimize it smartly, and let your qualifications work for you.
